Published: June 4, 2025
A team of researchers at Stanford University has developed a machine learning–driven strategy to re engineer the human protein zinc finger protein in a way that may reduce immune rejection risk in gene and cell therapies. The study, published in Cell Systems paper (Machine-guided dual-objective protein engineering for deimmunization and therapeutic functions). , introduces a new method to optimize protein functionality while minimizing the triggering adverse immune responses—an issue that remains in the immunology.
The work, led by Dr. Xiaojing Gao, assistant professor of chemical engineering, represents a careful blend of synthetic biology and computational protein design. Instead of introducing foreign proteins that the body may recognize as danger, the researchers aimed to design human-based proteins that less likely rejected by the immune system.
The Challenge of Immunogenicity in Therapeutics
Gene therapies like CAR-T and CRISPR hold great promise for treating a range of conditions—from cancer to rare genetic disorders. However, their effectiveness can be compromised when the immune system reacts to engineered components, particularly those derived from bacteria, virus or non-human organisms.
“We asked a simple question,” Dr. Gao explained. “Why not design therapies that are less likely to trigger an immune response from the start?”
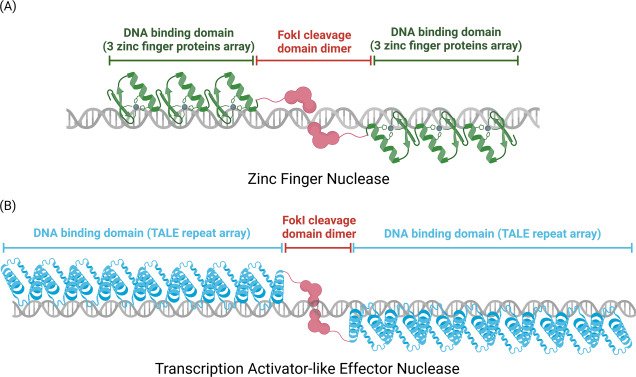
Their answer: Use proteins already found in the human body and redesign them to serve new therapeutic roles. That led them to zinc finger proteins—naturally occurring DNA-binding proteins with a long history in genetic regulation.
Designing Smarter Zinc Fingers
Zinc fingers are ideal candidates because they already play a role in regulating genes and are less likely to be flagged as foreign. But to use them effectively in gene therapy, they need to be customized to target new, specific locations in the genome.
To do this, Gao’s team developed a three-step machine learning framework:
- Target Matching: The first model predicts which zinc finger combinations can be assembled to bind a specific DNA sequence. This lets the team design custom “arrays” of zinc fingers capable of targeting virtually any genetic region.
- Immune Risk Screening: The second model evaluates the potential immunogenicity of the newly created junctions between individual zinc finger domains. These junctions don’t naturally exist in the body, so predicting whether they might trigger an immune response is critical. This model was trained on cancer vaccine data to estimate immune sensitivity.
- Function Optimization: Finally, the team applied a protein language model to fine-tune the functionality of the zinc fingers. This model suggested precise mutations to boost binding strength or stability—without increasing the risk of immune detection.
Each candidate design had to pass both the immune risk screen and the performance test before moving forward.
What the Results Showed
After building several candidate arrays, the team compared their performance to unmodified zinc fingers in lab tests. The original versions increased gene expression by two- to six-fold, while the AI-enhanced designs showed comparable or better performance, all while carrying a lower predicted risk of immune response.
“This isn’t about building a completely new tool,” said lead author Eric Wolsberg, a PhD student in chemical engineering. “It’s about making smarter use of the tools we already have—guided by AI.”
Implications for Future Therapeutics
This research demonstrates a practical way to reduce the immune risks that limit current therapeutic approaches. Instead of trial-and-error approaches that often face setbacks in clinical trials, this method integrates immune safety early in the design process.
While more testing is needed before clinical translation, the approach could help developers build safer, more personalized gene therapies in the near future.
Most importantly, this work underscores the growing role of AI in biotechnology—not to replace human scientists, but to enhance their ability to design safer, more effective treatments from the ground up.